Diabetes Diagnostic Tools
The objective of diabetes diagnostic tools is to assess an individual’s glucose levels and variability in order to develop a plan that will allow for proper management. The three most common diagnostic tools utilized by patients and physicians are A1C testing, self-monitoring blood glucose (SMBG), and continuous glucose monitoring systems (CGMS). Each type of testing provides certain types and quantities of data. We believe that CGMS will become the standard of care over time for type 1 and type 2 diabetic patients who rely on some insulin supplementation, while SMBG will continue to serve the type 2 patients who do not require insulin.
Glycemic goals provide a measure for success
Physicians set goals for patients A1C levels to be returned to as close to non-diabetic levels without subjecting individuals to complications of severe hypoglycemia or weight gain. With increased understanding of the disease, it has been determined that reducing A1C levels is associated with a reduction of the micro and macrovascular and neuropathic complications resultant of unmanaged diabetes. However, studies have shown (and common sense tells us) that more aggressive management of glucose levels using single-point glucose testing (but not CGMS) can lead to an exponential increase in hypoglycaemic events. With questions remaining regarding the benefits of intensive blood glucose regulation, current ADA recommendation is simply to reduce A1C tests to < 7%, as CGMS is newer to the diabetes community and is still being widely studied. The potential consequence of tighter glycemic control is the risk of hypoglycemic events that can result in death. A1C testing: looking through the rear view mirror
Physicians employ testing to confirm A1C levels for patients at regular intervals during a calendar year to ensure glycemic goals are being achieved. Patients with a fixed treatment protocol have A1C levels tested twice per year, and those that have had altered treatments receive quarterly A1C tests. Since the testing of A1C levels gives the physician a picture of the glycemic control achieved over the previous 120 days, a larger picture can be analyzed relative to the self monitored blood glucose records that provide a more finite assessment of disease management.
Self-monitor blood glucoses (SMBG) – single point testing: limited data provides limited control
Based on numerous clinical trials that show benefit, physicians reinforce that type 1 and insulin-dependent type 2 diabetics self-monitor blood glucose levels (SMBG) using finger-sticks at least three times daily. Physicians instruct blood glucose levels to be recorded based on the frequency of an individual’s insulin supplementation. The importance of self-monitoring is to prevent drastic changes in blood glucose levels and aid individuals in administering the best therapy regimen. Often, postprandial (time after a meal) levels are monitored to confirm the appropriate insulin is being delivered to the body immediately after an infusion of glucose into the body. Patients are educated to try to maintain blood glucose levels in the range of 70-150 mg/dl. Though there are immediate consequences for extreme blood glucose highs or lows, there are also the long-term concerns as poor overall glucose management can increase the risk of complications later in life.
For patients who are taking oral medication to manage their glucose levels (mostly type 2 diabetics), the importance of SMBG is not as clear as these patients often achieve their glucose goals in combination with diet and exercise. The 2009 American Diabetes Association guidelines regarding utilization of SMBG are as follows:
• SMBG should be carried out three or more times daily for patients using multiple insulin injections or insulin pump therapy
• For patients using less frequent insulin injections, non-insulin therapies, or medical nutrition therapy (MNT) and physical activity alone, SMBG may be useful as a guide to the success of therapy
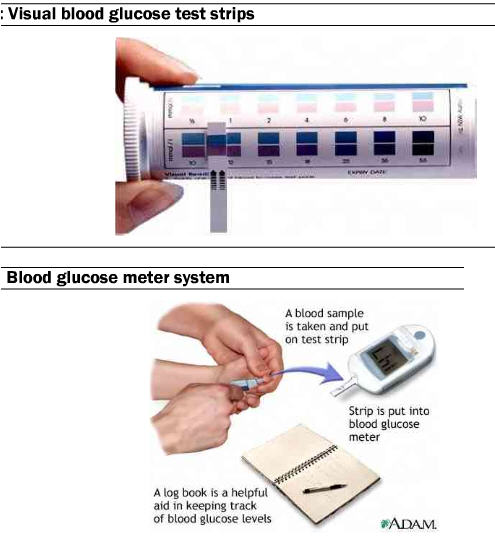
There are a variety of finger-stick devices available on the market, all of which try to mitigate discomfort and make results easy to read. The process involves the patient pricking a finger to draw a drop of blood which is placed on a test strip. Test strips can either give a visual reading (different colors for different glucose ranges), or the strip can be part of a kit that includes a meter than analyzes the test strip and displays the blood glucose levels.
It is estimated that the current worldwide market for SMBG fingerstick monitoring devices and disposables totals $10 billion, growing 11% annually. Four companies – Roche Diagnotics, LifeScan, Inc. (a division of Johnson & Johnson), Bayer Corporation and MediSense (a division of Abbott Laboratories) – currently account for over 90% of the worldwide sales of blood glucose self-monitoring systems.